Reductive Amination
More specifically – reaction as:
Less common and more challenging than imine reduction. This method does avoid the separate synthesis of an imine intermediate. However, as this must be achieved in situ the conditions required may be unfavourable to achieving subsequent reduction with high enantioselectivity. Use of a Brønsted acid or Lewis acid can aid imine formation under mild conditions, as can the use of molecular sieves. Aryl amines also aid the formation of ketimines/enamines. In addition, in situ imine formation may result in a mixture of E and Z isomers, and the reduction reaction needs to be chemoselective (imine vs. ketone reduction). Reaction of the amine product in a second reductive amination may be a problem. These issues notwithstanding, some very useful methodologies have been developed, albeit limited to a relatively narrow range of substrates, and using ligands for which both enantiomers are not always available. Links to the ligands used in the methodologies described are within the text.
Reviews: TCC14-343-261, OCF21-8-2328.
i) With ammonia/ammonium salts.
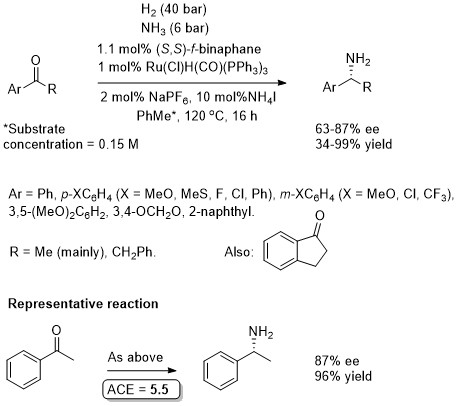
Reaction conditions are not applicable to dialkyl (low ee – but see EJOC20-4796 – below) or diaryl ketones (no reaction). (S)-f-Binaphane is commercially available.
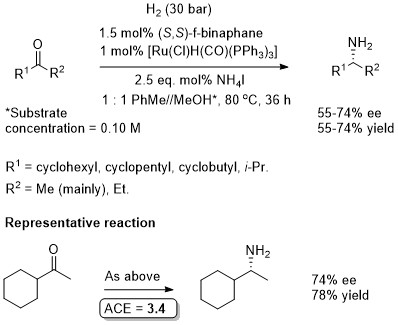
Where R1 = n-alkyl, ee values 21-33%. Where R1 = t-alkyl, poor conversion. With acetophenone, ee = 93%, yield = 65%, i.e. a higher selectivity (but lower yield) to that obtained using the conditions reported in JACS18-140-355 (above). (S)-f-Binaphane is commercially available.
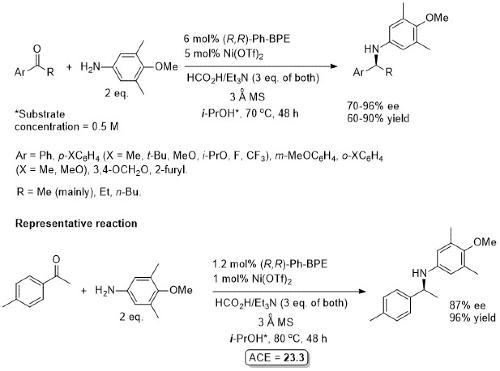
ii) With primary amines
Both (R,R) and (S,S) Ph-BPF ligands available. Formic acid used as an alternative to hydrogen. An anisidine was used as the aromatic amine as oxidative deprotection of the product may be used to generate the corresponding primary amine. Aliphatic methyl ketones gave low ee (<20%).
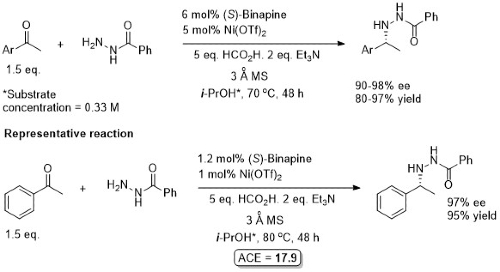
The reaction was also adapted to the use of benzhydrazide as amine source:
(S)-Binapine (but not R ) commercially available. The N-N bond of the products can be cleaved to give the corresponding primary amine with SmI2 or Raney nickel. The N-benzoylhydrazine products are crystalline.
For two aliphatic ketones a Josiphos ligand gave good ee:
There are some significant differences between the paper and the SI with respect to the reaction conditions/equivalents. SI used for the above.
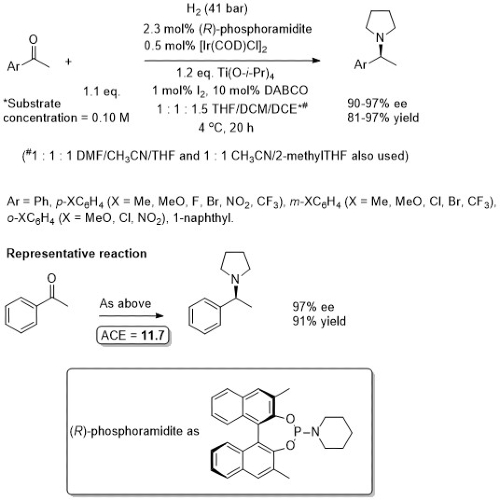
iii) With secondary amines
CS19-10-4509 (open access)
This phosphoramidite does not appear to be commercially available, but both enantiomers (R) and (S) of the precursor 3,3’-dimethylBINOL are. Other dialkyl secondary amines were employed with similar ee values using a related ligand (MeO in place of Me, (S)-MeNCH(Me)Ph in place of piperidine). In the absence of the 3,3’-substituents the ee value is low (24% for the example used in the representative reaction, with NEt3 in place of DABCO).