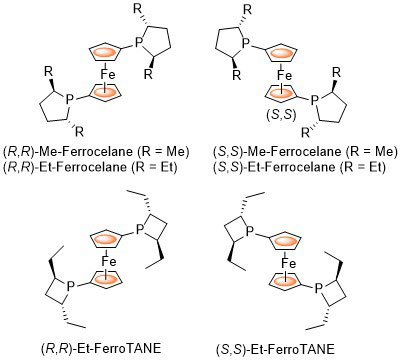
Ferrocelane ligands were first reported by Mark Burk (Duke University) as 1,1’-ferrocene based equivalents of DuPHOS ligands. FerroTANE ligands were later reported essentially simultaneously by Marinetti (Paris) and Burk (then at Chirotech). The following lists reactions, organised by metal (Pd, Rh, Ru, Ir, Co, Cu) for which the application of these ligands has resulted in >80% ee. ACE = Asymmetric Catalytic Efficiency. Literature covered until end 2019 (14 entries).
Palladium
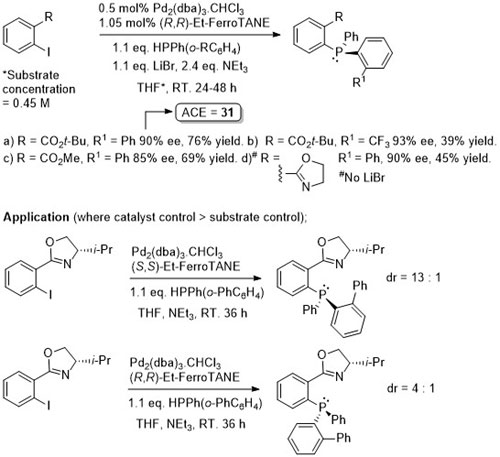
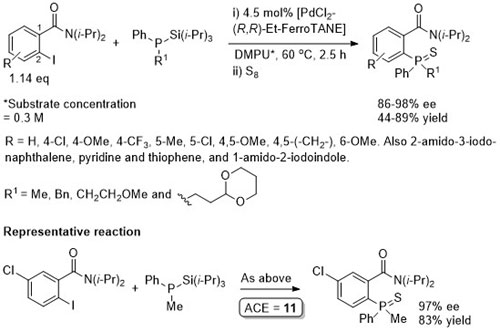
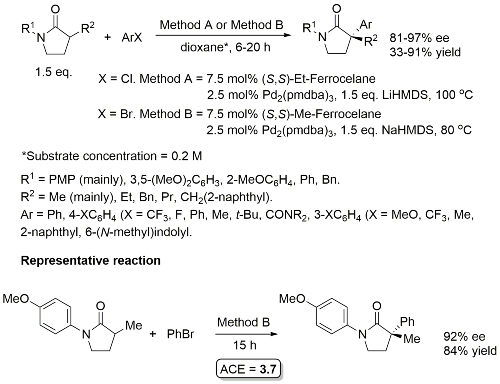
Pd – C-P Coupling CC04-530
Absolute configuration of products not determined, but may possibly be inferred from CC04-530 as the reaction described also proceeds via an intermediate palladacycle, and assuming rapid epimerisation (relative to reductive elimination) of intermediate Pd(II)-phosphide complexes mediated by Et-FerroTANE.
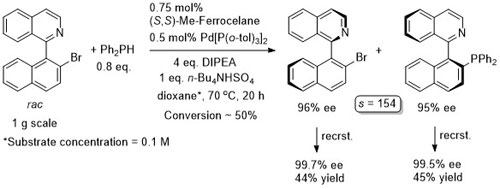
Pd – C-C Coupling Angew19-58-4297
Only a trace of product observed with ortho-substituted aryl halides under these conditions.
Rhodium
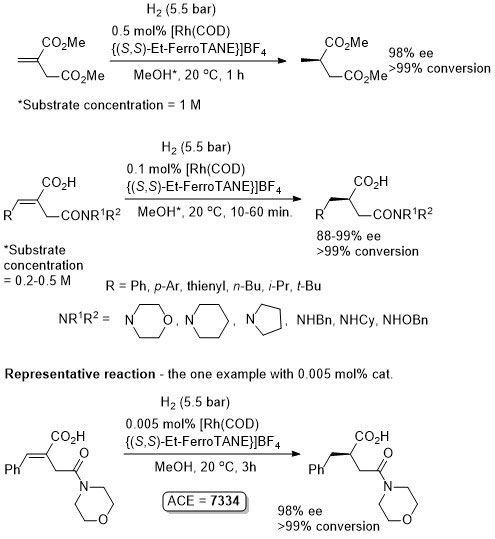
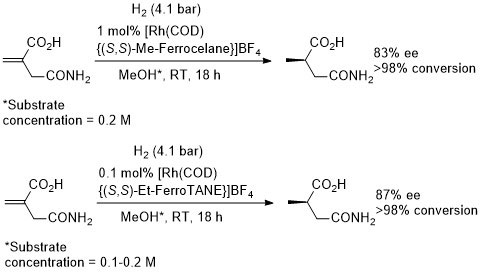
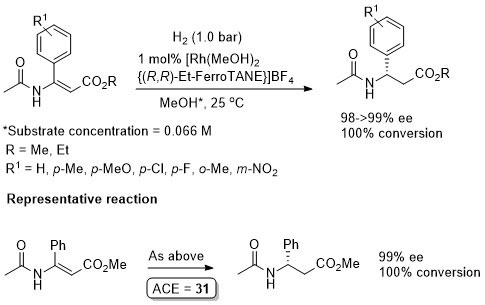
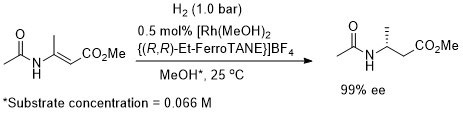
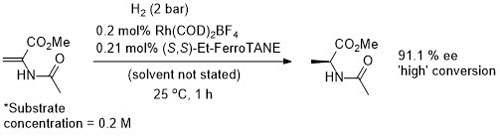
Rh – Hydrogenation TL94-35-9363
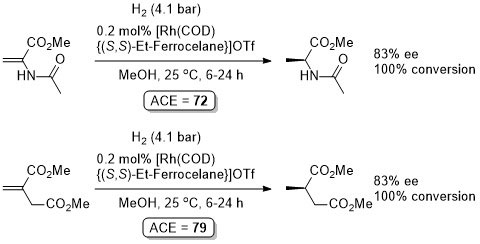
Absolute configuration not stated but assigned as S by comparison to results in OL02-4-4471.
These results were obtained during a screening and optimisation study on the asymmetric hydrogenation of 2-methylenesuccinamic acid. Catalyst [Rh(COD){(S,S)-Et-DuPHOS}]BF4 was further optimised to give R product in 96% ee and >98% conversion with S/C = 100000 : 1 provided the substrate was chloride free (ACE = 18750).
Reaction times not stated but pseudo-first-order half-times given as between 0.5 (Ar = Ph) and 1.8 min (Ar – m-nitrophenyl). Catalyst loading also reduced to 0.1 mol% but ee not stated. High activity possibly due to large P-Rh-P bite angle (98.3o – contrast with approx. 85o in corresponding DuPHOS complexes.
Conversion/yield and reaction time not stated but pseudo-first-order half-time given as 1.2 min. Absolute configuration not stated and assigned tentatively by comparison to the above examples (i.e. Angew03-42-913).
Absolute configuration not stated and assigned by comparison to results reported in TL94-35-9363 and Angew00-39-1981 (described above). Reported ee an average of 32 simultaneous runs (using both enantiomers of the ligand) performed in a parallel high pressure microreactor.
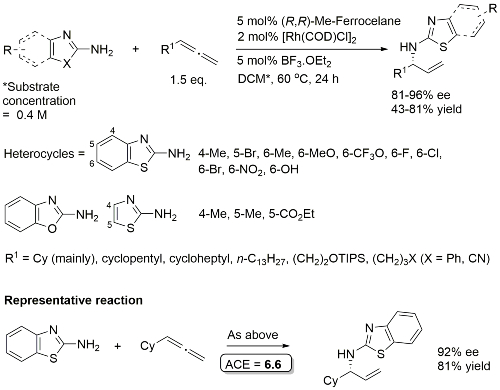
Rh – Hydroamination EJOC19-5180
High regioselectivity for -NH2 vs. N allylation. 1 : 1 DCM/EtOH used for 6-NO2 and 6-OH examples. One example with a 1,1-disubstituted allene (R1 = EtOCOCH2 R2 = Me) to give a quaternary stereocentre. Same sense of absolute configuration (with Me replacing H).
Ruthenium
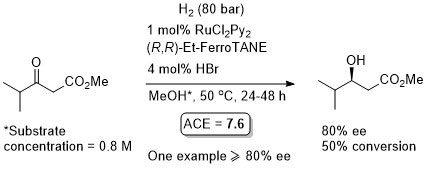
Ru – Hydrogenation EJIC03-2583
Iridium
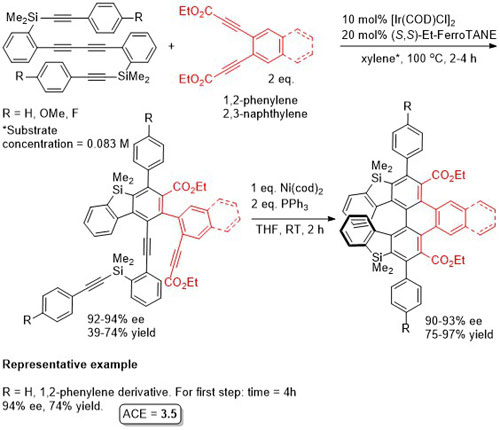
Ir – [2 + 2 + 2] cycloaddition CC12-48-1311
Cobalt
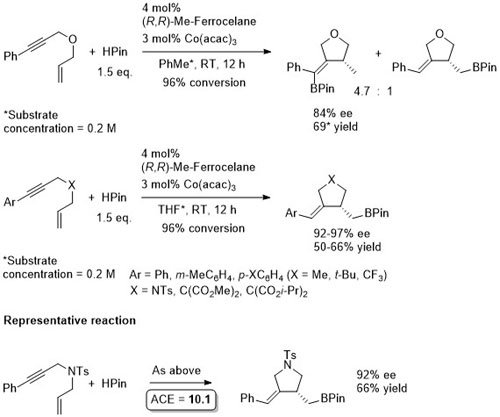
Co – Hydroboration – 1,6-enyne cyclisation JACS17-139-6526
The first scheme above is the result of a ligand scoping study for which (R,R)-QuinoxP was identified as the best ligand (under the same conditions the same enantiomer of the product was formed with a 49:1 chemoselectivity, 99% ee and 85% yield). Observed that N- and C-tethered enynes underwent hydroboration/cyclisation to alkyl boronate esters with Me-Ferrocelane as ligand in THF (2nd and 3rd schemes).
Copper
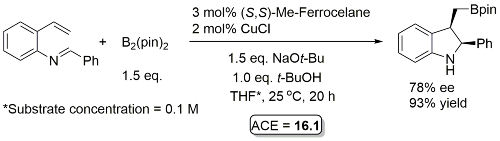
Cu – Hydroboration/cyclisation OL18-20-1798
Not 80% ee but close and an interesting reaction. Result from a ligand scoping study from which under the same conditions (S,S)-Ph-BPE gave 81% ee (enantiomeric product), 98% yield. Reaction further optimised with (S,S)-Ph-BPE identifying MTBE as a better solvent (89% ee, 97% yield) and exemplified with this solvent with a series of imines derived from aryl aldehydes.