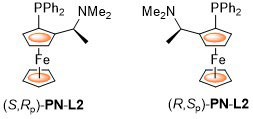
The original. First synthesised by Hayashi, Yamamoto and Kumada using the diastereoselective lithiation of N,N-dimethyl-1-ferrocenylethylamine reported previously by Ugi et al. PPFA has principally been employed as an intermediate in the synthesis of many other ligands, there being relatively few reports on the use of this as a ligand in asymmetric catalysis. Examples of Pd, Cu and Ag catalysis that result in >80% ee are listed below. ACE = Asymmetric Catalytic Efficiency. Literature covered until end 2017 (12 entries).
Palladium
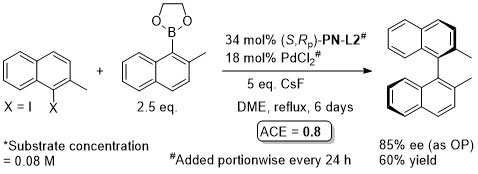
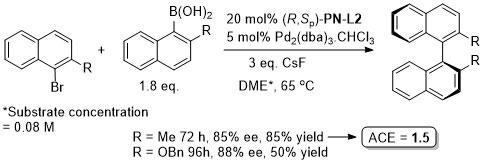
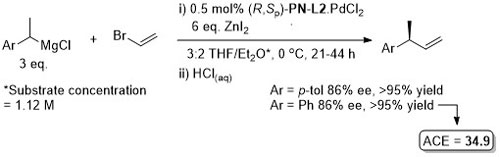
Pd – Asymmetric Suzuki cross-coupling CC00-1723 and Tet04-60-4377
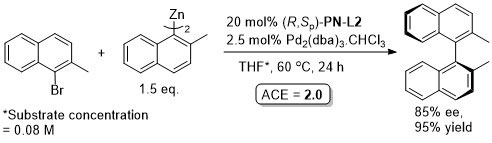
Pd – Asymmetric Negishi cross-coupling TA06-17-2593
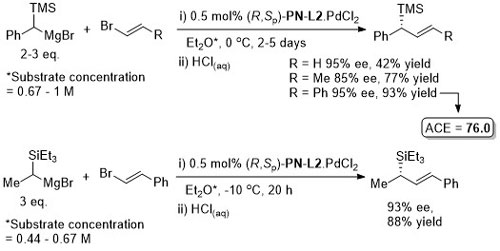
Pd – Asymmetric Grignard cross-coupling JOC86-51-3772
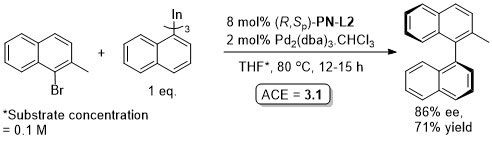
Pd – Asymmetric organoindium cross-coupling EJOC13-2555
Copper
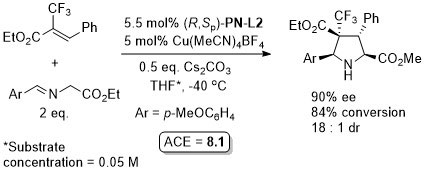
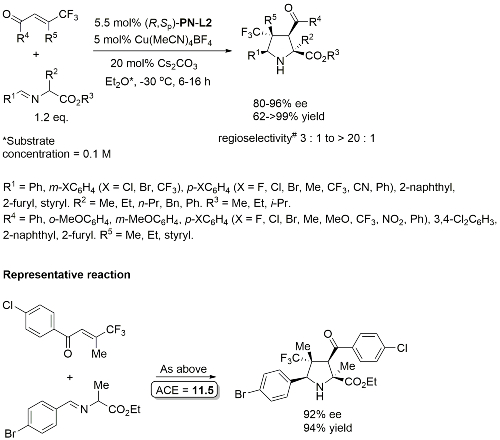
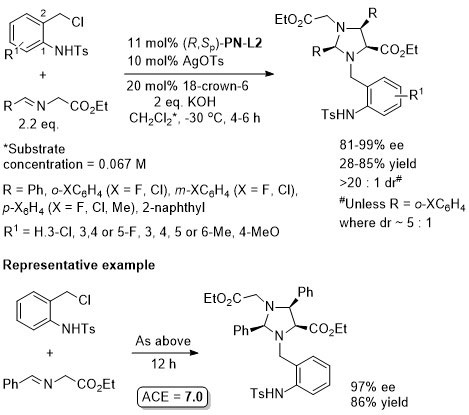
Cu – 3 + 2 cycloaddition ACSCat17-7-210
One example with (S,Sp)-PN-L2 from a scoping study in which a Ming-Phos ligand gave a similar result (94% ee, 91% conversion, 15 : 1 dr at -40 oC). Further reaction optimisation and exemplification was with a Ming-Phos ligand.
Methodology also applicable to a β-CHF2 or a β-CH2F substituent in place of CF3, but unsuccessful where CF3 replaced by CCl3 or Me. Use of (R,Sp)-PN-L1a under the same conditions resulted premoninantly in an alternative regioisomer (#). See entry under (S,Sp)-PN-L1a. Rationalised as a result of differeing coordination modes, with PN-L1a = bidentate and PN-L2 = pseudobidentate.
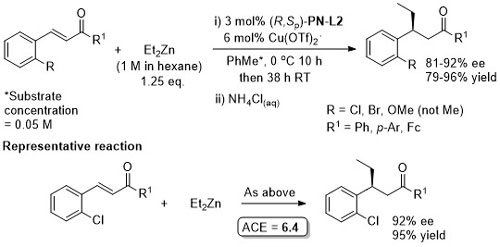
Cu – Imine alkylation TA05-16-2531
Cu – Conjugate addition TA06-17-136
Absolute configuration of products assigned by use of data in TA12-23-130
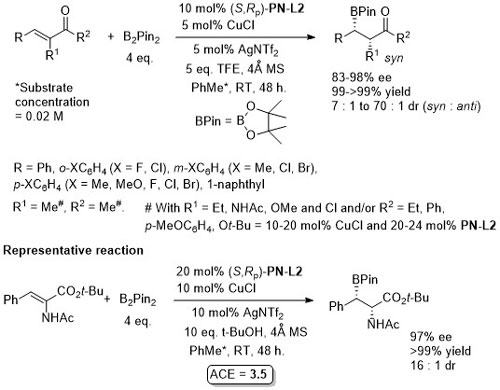
Cu – Beta (1,4) borylation OL16-18-3926
Silver salt rather than base used to avoid α-carbonyl epimerisation. Use of Phosferrox PN-L1a and Josiphos-1 reported to result in no product formation.
Silver
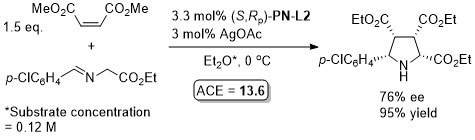
Ag – 3 + 2 cycloaddition JACS07-129-750
Not an example of >80% ee but included as the corresponding ligand with NMe2 replaced by NH2 results in a hydrogen-bonding reversal of enantioselectivity (83% ee).
Ag – 3 + 2 cycloaddition and 1,4-addition OL17-19-5236