Scopus Profile Web of Science Profile Google Profile
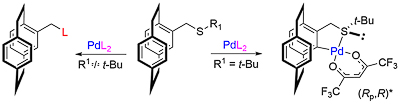
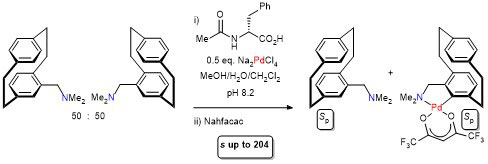
103. The Multifaceted Chemistry of [2.2]Paracyclophane-Based Thioethers with Palladium(II) Complexes
D. Deschamps, H. Gazzeh, A. Bonciani, C. J. Richards. A.-C. Gaumont and S. Perrio, Eur. J. Org. Chem. 2024, 27, e202301181.
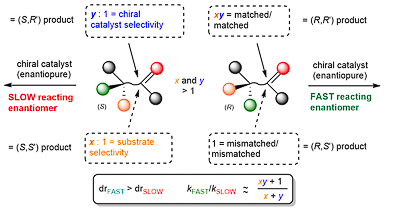
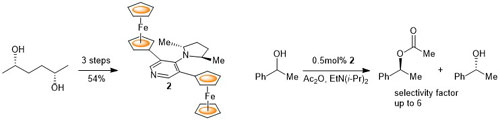
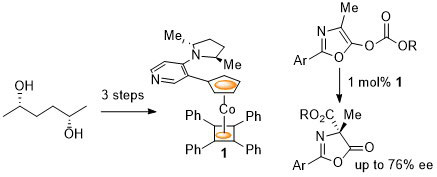
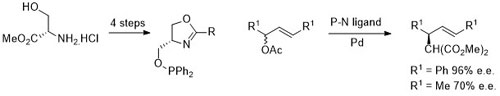
102. Double Asymmetric Synthesis: Faster Reactions are More Selective and a Model to Estimate Relative Rate.
C. J. Richards and O. Stephen Ojo, Org. Biomol. Chem. 2023, 21, 7115-7128. [open access]
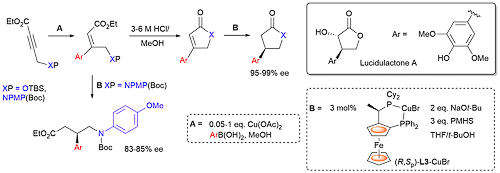
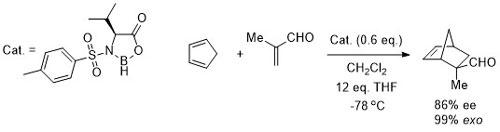
101. An Expedient Copper-Catalysed Asymmetric Synthesis of γ-Lactones and γ-Lactams. Application to the Synthesis of Lucidulactone A.
O. Stephen Ojo, D. L. Hughes and C. J. Richards, Org. Biomol. Chem. 2023, 21, 4144-4149. [open access]
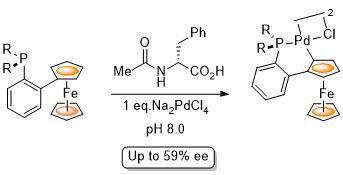
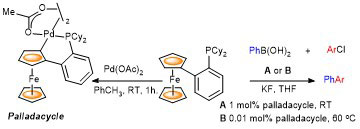
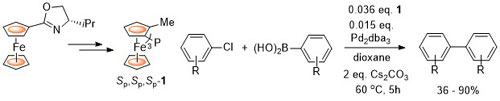
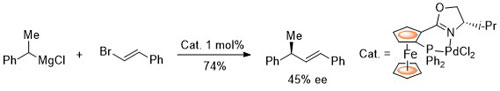
100. Application of Ferrocene-Based Palladacycle Precatalysts to Enantioselective Aryl-Aryl Kumada Coupling.
R. A. Arthurs, D. L. Hughes and C. J. Richards, Eur. J. Inorg. Chem. 2022, e202101077. [open access]
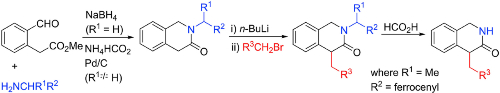
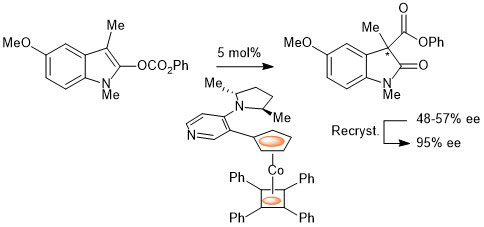
99. Synthetic approaches to N- and 4-substituted 1,4-dihydro-3(2H)-isoquinolinone derivatives.
M. J. O’Sullivan, R. J. D. Hatley, C. R. Wellaway, S. P. Bew and C. J. Richards, Tetrahedron 2021, 100, 132455. [PDF of accepted manuscript]
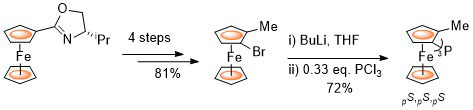
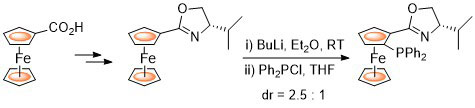
98. Copper(I) Complexes of P-Stereogenic Josiphos and Related Ligands
R. A. Arthurs, A. C. Dean, D. L. Hughes and C. J. Richards, Eur. J. Org. Chem. 2021, 2719-2725. [open access]
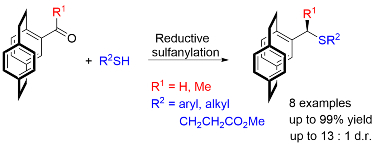
97. Functionalisation of [2.2]Paracyclophanes via a Reductive Sulfanylation Reaction
D. Deschamps, J.-F. Lohier, C. J. Richards, A.-C. Gaumont and S. Perrio, J. Org. Chem. 2021, 86, 507. [PDF of accepted manuscript]
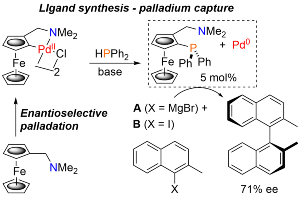
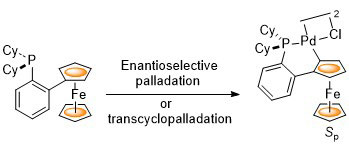
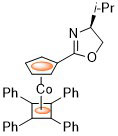
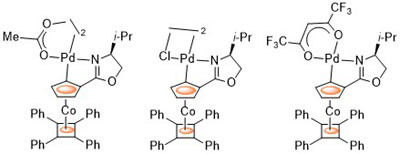
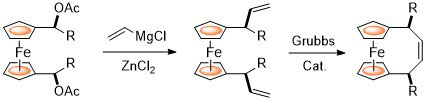
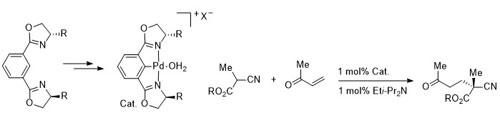
96. Planar Chiral Palladacycle Precatalysts for Asymmetric Synthesis.
R. A. Arthurs, D. L. Hughes and C. J. Richards, Org. Biomol. Chem. 2020, 18, 5466. [open access]
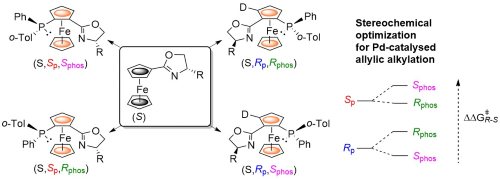
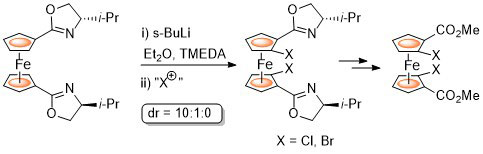
95. Stereoselective Synthesis of all Possible Phosferrox Ligand Diastereoisomers Displaying Three Elements of Chirality: Stereochemical Optimization for Asymmetric Catalysis.
R. A. Arthurs, D. L. Hughes and C. J. Richards, J. Org. Chem. 2020, 85, 4838. [PDF of accepted manuscript]
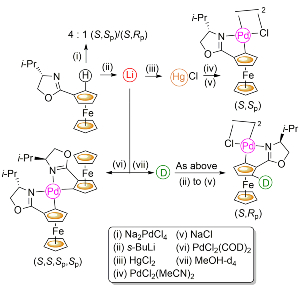
94. Ferrocenyloxazoline-Derived Planar Chiral Palladacycles: C-H Activation, Transmetalation, and Reversal of Diastereoselectivity.
R. A. Arthurs, D. L. Hughes and C. J. Richards, Organometallics, 2019, 38, 4271. [PDF of accepted manuscript]
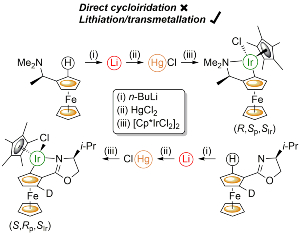
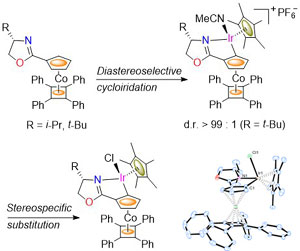
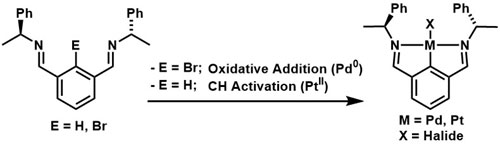
93. Application of Transmetallation to the Synthesis of Planar Chiral and Chiral-at-Metal Iridacycles.
R. A. Arthurs, D. L. Hughes, P. N. Horton, S. J. Coles and C. J. Richards, Organometallics, 2019, 38, 1099. [PDF of accepted manuscript]
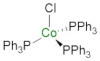
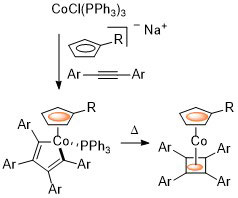
92. Chlorotris(triphenylphosphine)cobalt
C. J. Richards, e-EROS Encyclopedia of Reagents for Organic Synthesis 2019.
91. Enantiopure Planar Chiral and Chiral-at-Metal Iridacycles Derived from Bulky Cobalt Sandwich Complexes.
R. A. Arthurs, C. C. Prior, D. L. Hughes, V. S. Oganesyan and C. J. Richards, Organometallics, 2018, 37, 4204. [PDF of accepted manuscript]
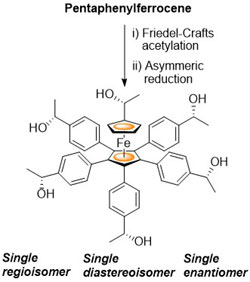
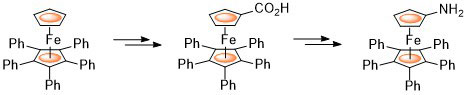
90. Multiple Acetylation of Pentaphenylferrocene. Synthesis and Asymmetric Reduction of 1-Acetyl-1′,2′,3′,4′,5′-penta(para-acetylphenyl)ferrocene.
R. A. Arthurs and C. J. Richards Eur. J. Inorg. Chem. 2018, 1655. [PDF of accepted manuscript]
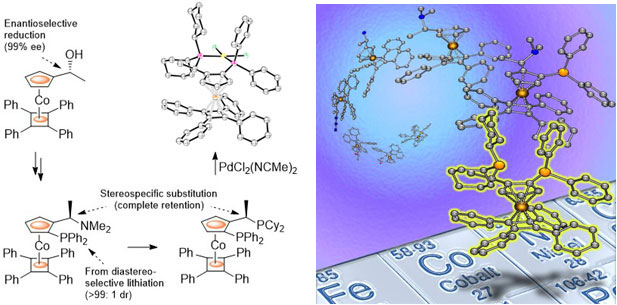
89. Stereoselective and Stereospecific Reactions of Cobalt Sandwich Complexes. Application to the Synthesis of a New Class of Single Enantiomer Bulky Planar Chiral P-N and P-P Ligands
R. A. Arthurs, P. N. Horton, S. J. Coles and C. J. Richards Chem. Eur. J. 2018, 24, 4310. [PDF of accepted manuscript]
88. Synthesis of Diastereomeric Bis(oxazoline) Ligands Derived from (S,S)-1,1′-Bis(4-isopropyloxazolin-2-yl)ferrocene.
R. A. Arthurs and C. J. Richards Synlett 2018, 29, 585. [PDF of accepted manuscript]
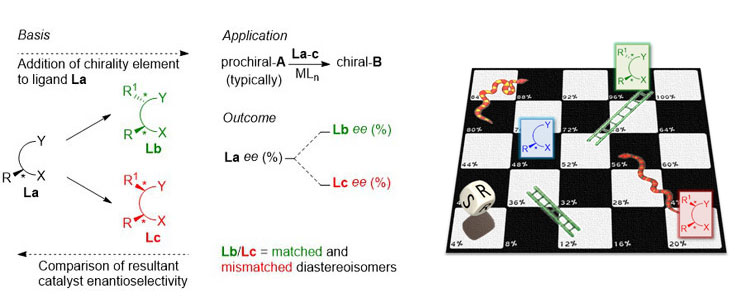
87. Catalyst Optimisation for Asymmetric Synthesis by Ligand Chirality Element Addition – A Perspective on Stereochemical Cooperativity.
C. J. Richards and R. A. Arthurs, Chem. Eur. J. 2017, 48, 11460. [PDF of accepted manuscript]
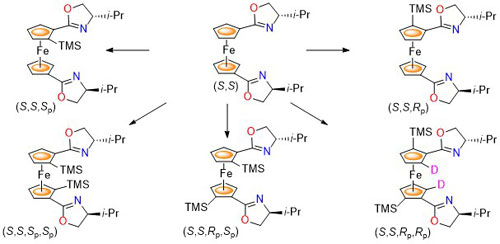
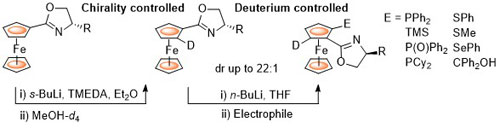
86. Deuterium as a Stereochemically Invisible Blocking Group for Chiral Ligand Synthesis.
R. A. Arthurs and C. J. Richards, Org. Lett. 2017, 19, 702. [PDF of accepted manuscript]
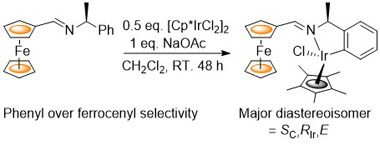
85. Phenyl vs. Ferrocenyl Cyclometallation Selectivity: Diastereoselective Synthesis of an Enantiopure Iridacycle.
R. A. Arthurs, P. N. Horton, S. J. Coles and C. J. Richards, Eur. J. Inorg. Chem. 2017, 229. [PDF of accepted manuscript]
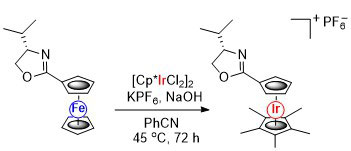
84. Metallocene to Metallocene Conversion. Synthesis of an Oxazoline-Substituted Pentamethyliridocenium Cation from a Ferrocenyloxazoline.
R. A. Arthurs, P. N. Horton, S. J. Coles and C. J. Richards, Chem. Commun. 2016, 52, 7024-7027. [PDF of accepted manuscript]
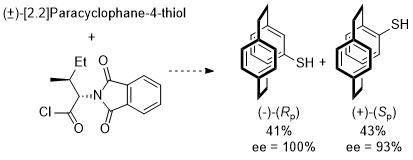
83. Enantiomerically Pure [2.2]Paracyclophane–4–thiol: A Planar Chiral Sulfur-based Building Block Readily Available by Resolution with an Amino Acid Chiral Auxiliary.
A. Vincent, D. Deschamps, T. Martzel, J.-F. Lohier, C. J. Richards, A.-C. Gaumont and S. Perrio, J. Org. Chem. 2016, 81, 3961-3966. [PDF of accepted manuscript]
82. Enantiopure ferrocene-based planar-chiral iridacycles: stereospecific control of iridium-centred chirality.
R. A. Arthurs, M. Ismail, C. C. Prior, V. S. Oganesyan, P. N. Horton, S. J. Coles and C. J. Richards, Chem. Eur. J. 2016, 22, 3065-3072. [PDF of accepted manuscript]
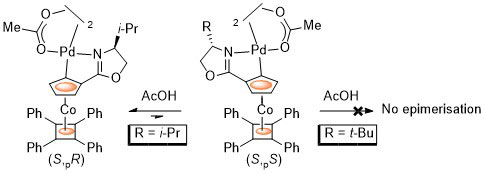
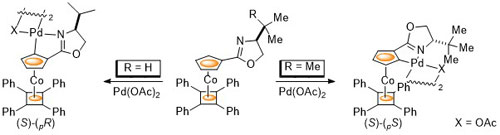
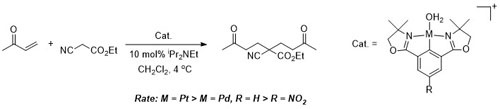
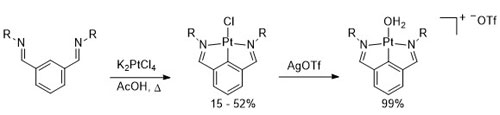
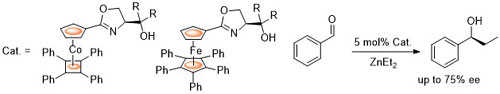
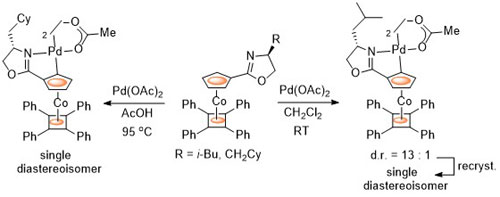
81. Chirality Control in Planar Chiral Cobalt Oxazoline Palladacycles.
D. J. Cassar, H. Roghzai, D. Villemin, P. N. Horton, S. J. Coles and C. J. Richards, Organometallics 2015, 34, 2953-2961. [Link to accepted manuscript]
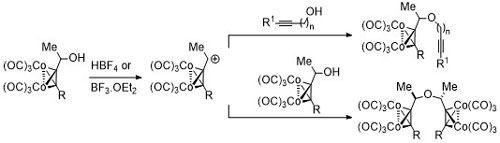
80. Application of the Nicholas reaction to the synthesis of dicobalt hexacarbonyl complexed diyne ethers.
J. Amin, M. Motevalli and C. J. Richards, J. Organomet. Chem. 2015, 776, 43-50.
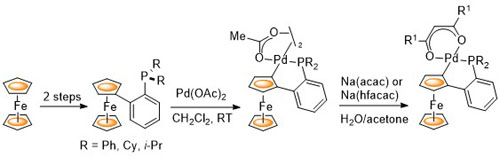
79. Synthesis of racemic palladacycles from 2-ferrocenylphenylphosphines.
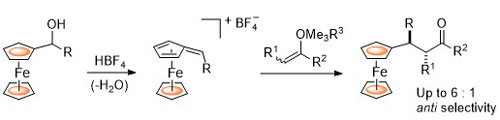
K. Panchal, J. Amin, F. X. Roca, M. Motevalli, P. N. Norton, S. J. Coles and C. J. Richards, J. Organomet. Chem. 2015, 775, 12-19.
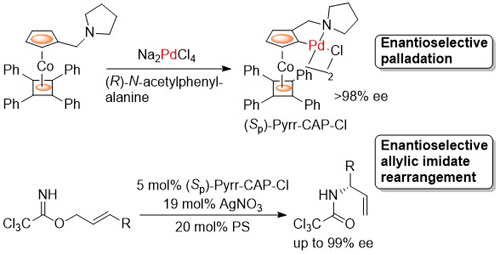
78. Enantioselective Synthesis and Application to the Allylic Imidate Rearrangement of Amine-Coordinated Palladacycle Catalysts of Cobalt Sandwich Complexes.
D. J. Cassar, G. Ilyashenko, M. Ismail, J. Woods, D. L. Hughes and C. J. Richards, Chem. Eur. J. 2013, 19, 17951-17962. [open access]
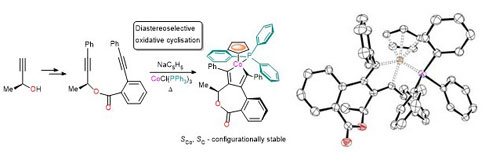
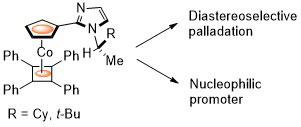
77. Diastereoselective Synthesis of Half-Sandwich Chiral-at-Metal Cobaltacycles by Oxidative Cyclisation
J. Amin and C. J. Richards, Chem. Commun. 2012, 48, 10192-10194. [open access]
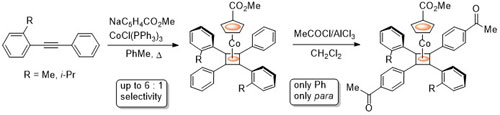
76. Regioselective, Stereoselective and Conformationally Controlled Synthesis of (η4-Tetraarylcyclobutadiene)(η5-carbomethoxy-cyclopentadienyl)cobalt Metallocenes
D. Cassar, E. Nagaradja, D. C. D. Butler, D. Villemin and C. J. Richards, Org. Lett. 2012, 14, 894-897.
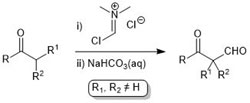
75. α-Formylation of α-Substituted Ketones
Jamal Hassan and Christopher J. Richards, Synlett 2012, 23, 239-242.
74. Synthesis of a [2.2]Paracyclophane Based Planar Chiral Palladacycle by a Highly Selective Kinetic Resolution/C-H Activation Reaction
N. Dendele, F. Bisaro, A.-C. Gaumont, S. Perrio and C. J. Richards, Chem. Commun. 2012, 48, 1991-1993.
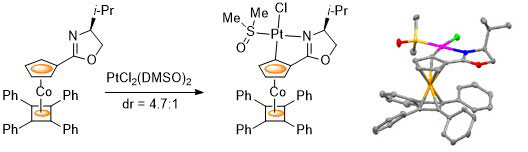
73. Diastereoselective Synthesis of Planar Chiral Cobalt Metallocene based Oxazoline Platinacycles
Emin Günay, David. L. Hughes and Christopher J. Richards Organometallics 2011, 30, 3901-3904.
72. Size Does Matter. Sterically Demanding Metallocene-Substituted 3-Methylidene-Oxindoles Exhibit Poor Kinase Inhibitory Action
J. Spencer, J. Amin, P. Coxhead, J. McGeehan, C. Richards, G. J. Tizzard, S. J. Coles, J. Bingham, J. Hartley, L. Feng, E. Meggers and M. Guille Organometallics 2011, 30, 3177-3181.
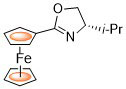
71. (S)-(-)-[4,5-Dihydro-4-(1-methylethyl)-2-oxazolinyl]ferrocene
C. J. Richards, e-EROS Encyclopedia of Reagents for Organic Synthesis 2011.
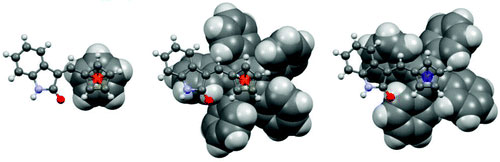
70. Models for the Basis of Enantioselection in Palladium Mediated C-H Activation Reactions
E. Günay and C. J. Richards, Tetrahedron: Asymmetry 2010, 21, 2782.
69. Suzuki Reactions for the Desymmetrization of 1,2- and 1,3-bulky Cobalt Metallocenes
Bergin, D. Hughes and C. J. Richards, Tetrahedron: Asymmetry 2010, 21, 1619-1623.
68. Planar Chiral Palladacycle Catalysed Allylic Imidate Rearrangements
Nomura and C. J. Richards, Chem. Asian J. 2010, 5, 1726-1740.
67. Metallocyclic Ferrocenyl Ligands. C. J. Richards, Chiral Ferrocenes in Asymmetric Catalysis
ed. L.-X. Dai and X.-L. Hou, Wiley-VCH, Weinheim, 2010, ch12, pages 337-368.
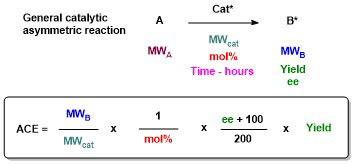
66. Can We Measure Catalyst Efficiency in Asymmetric Chemical Reactions? A Theoretical Approach
S. El-fayyoumy, M. H. Todd and C. J. Richards, Beil. J. Org. Chem. 2009, 5, No. 67. [open access]
65. Synthesis of Planar Chiral Phosphapalladacycles by N-Acyl Amino Acid Mediated Enantioselective Palladation
E. Günay and C. J. Richards, Organometallics 2009, 28, 5833.-5836.
64. Asymmetric Synthesis of Oxindoles Containing a Quaternary Stereogenic Centre by Catalytic O/C-carboxyl Rearrangement
M. Ismail, H. V. Nguyen, G. Ilyashenko, M. Motevalli and C. J. Richards, Tetrahedron Lett. 2009, 50, 6332-6334.
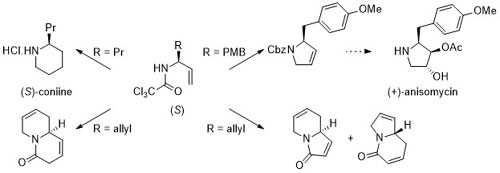
63. Asymmetric Synthesis of Unsaturated Monocyclic and Bicyclic Nitrogen Heterocycles
H. Nomura and C. J. Richards, Org. Lett. 2009, 11, 2892-2895. [PDF of accepted manuscript]
62. Synthesis and 1H NMR Spectroscopic Properties of Substituted (η4-tetraarylcyclobutadiene)(η5-cyclopentadienyl)cobalt Metallocenes
H. V. Nguyen, M. R. Yeamine, J. Amin, M. Motevalli and C. J. Richards, J. Organomet. Chem. 2008, 693, 3668-3676.
61. (η4-Tetraphenylcyclobutadiene)(η5-cyclopentadienyl)cobalt
C. J. Richards, e-EROS Encyclopedia of Reagents for Organic Synthesis 2008.
60. An Investigation into the Diastereoselective Palladation of Oxazoline Appended Cobalt Metallocenes
R. Yeamine and C. J. Richards, Tetrahedron: Asymmetry 2007, 18, 2613-2616.
59. An Investigation into the Allylic Imidate Rearrangement of Trichloroacetimidates Catalysed by Cobalt Oxazoline Palladacycles
H. Nomura and C. J. Richards, Chem. Eur. J. 2007, 13, 10216-10224
58. Synthesis and Crystal Structures of the First C2-Symmetric Bis-aldimine NCN-Pincer Complexes of Platinum and Palladium
S. Fossey, M. L. Russell, K. M. A. Malik and C. J. Richards, J. Organomet. Chem. 2007, 692, 4843-4848.
57. Chiral Pincer Complexes and Their Application to Asymmetric Synthesis
C. J. Richards and J. S. Fossey, in The Chemistry of Pincer Type Complexes, ed D. Morales-Morales and C. M. Jensen, Elsevier, Amsterdam, 2007, ch 3, pages 45-78.
56. Preparation of (η5-(S)-2-(4-Methylethyl)oxazolinylcyclopentadienyl)(η4-tetraphenylcyclobutadiene)cobalt
E. Anderson, L. E. Overman, C. J. Richards, M. P. Watson and N. White. Organic Syntheses 2007, 84, 139-147.
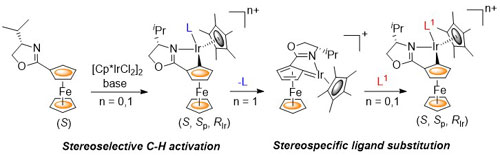
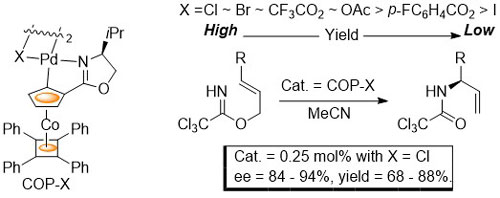
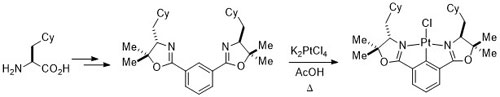
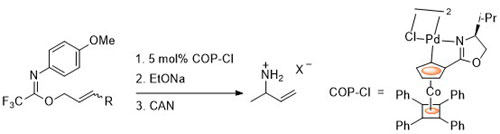
55. Preparation of the COP Catalysts: [(S)-COP-OAc]2, [(S)-COP-Cl]2, and (S)-COP-hfacac
E. Anderson, S. F. Kirsch, L. E. Overman, C. J. Richards and M. P. Watson, Organic Syntheses 2007, 84, 148-155.
54. A C2-Symmetric Metallocene-Pyrrolidinopyridine Nucleophilic Catalyst for Asymmetric Synthesis
H. V. Nguyen, M. Motevalli and C. J. Richards, Synlett 2007, 725-728.
53. A Metallocene-Pyrrolidinopyridine Nucleophilic Catalyst for Asymmetric Synthesis
D. C. D. Butler, H. V. Nguyen and C. J. Richards, Org. Lett. 2006, 8, 769-772.
52. (R)-2-Ferrocenyl-4-hydroxymethyl-4,5-dihydro-1,3-oxazole
M. H. Todd, M. Motevalli, S. El-Fayyoumy, C. Richards, Acta Cryst. 2006, E62, M719-720.
51. Synthesis of Planar Chiral Cobalt Metallocenes by Microwave-Assisted Diastereoselective Complexation
C. J. Taylor, M. Motevalli and C. J. Richards, Organometallics 2006, 25, 2891-2902.
50. Exploiting Planar Chirality for the Generation of α-Ferrocenyl Stereogenic Centres
C. J. Taylor, F. X. Roca and C. J. Richards, Synlett 2005, 2159-2162.
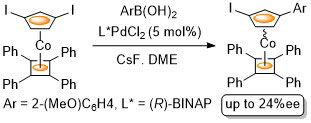
49. Synthesis of Planar Chiral Phosphapalladacycles by Highly Enantioselective Transcyclometallation
F. X. Roca, M. Motevalli and C. J. Richards, J. Am. Chem. Soc. 2005, 127, 2388-2389.
48. Synthesis of tert-Leucine Derived Cobalt Oxazoline Palladacycles. Reversal of Diastereoselective Palladation Selectivity and Application to the Asymmetric Rearrangement of N-Aryl Trifluoroacetimidates
R. S. Prasad, C. E. Anderson, C. J. Richards, L. E. Overman, Organometallics 2005, 24, 77.
47. Direct Platination as a Route to Conformationally Restricted Enantiopure C2-Symmetric Pincer Complexes
J. S. Fossey, G. Jones, H. V. Nguyen, C. J. Richards, M. A. Stark and H. V. Taylor, Tetrahedron: Asymmetry 2004, 15, 2067.
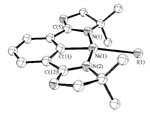
46. Synthesis and X-ray Crystal Structure Analysis of the First Nickel Bisoxazoline Pincer Complex.
J. S. Fossey and C. J. Richards, J. Organmet. Chem. 2004, 689, 3056.
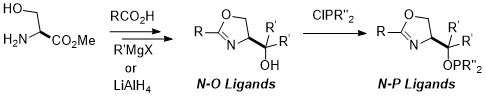
45. Serine Derived N-O and N-P Oxazoline Ligands for Asymmetric Catalysis
G. Jones and C. J. Richards, Tetrahedron: Asymmetry 2004, 15, 653.
44. Synthesis of 2,6-Bis(2-oxazolinyl)phenylplatinum(II) NCN Pincer Complexes by Direct Cyclometallation. Catalysts for Carbon-Carbon Bond Formation
J. S. Fossey and C. J. Richards, Organometallics 2004, 23, 367.
43. A Ferrocene Based Palladacyclic Precatalyst for the Suzuki Cross-Coupling of Aryl Chlorides
F. X. Roca and C. J. Richards, Chem. Commun. 2003, 3002.
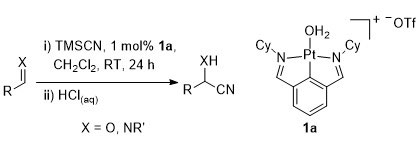
42. Catalysis of Aldehyde and Imine Silylcyanation by Platinum and Palladium NCN-Pincer Complexes
J. S. Fossey and C. J. Richards, Tetrahedron Lett. 2003, 44, 8773.
41. Catalytic Asymmetric Rearrangement of Allylic N-Aryl Trifluoroacetimidates. A Practical Method for Transforming Prochiral Allylic Alcohols to Chiral Allylic Amines
L. E. Overman, C. E. Owen, M. M. Pavan and C. J. Richards, Org. Lett. 2003, 5, 1809.
40. Synthesis of Monodentate Ferrocenylphosphines and Their Application to the Palladium Catalysed Suzuki Reaction of Aryl Chlorides
T. E. Picket, F. X. Roca and C. J. Richards, J. Org. Chem. 2003, 68, 2592.
39. A Direct Route to Platinum NCN-Pincer Complexes Derived From 1,3-bis(imino)benzenes and an Investigation into Their Activity as Catalysts for Carbon-Carbon Bond Formation.
J. S. Fossey and C. J. Richards, Organometallics 2002, 21, 5259.
38. The Synthesis of 1′-Substituted Derivatives of 1,2,3,4,5-Pentaphenylferrocene
D. C. D. Butler and C. J. Richards, Organometallics 2002, 21, 5433.
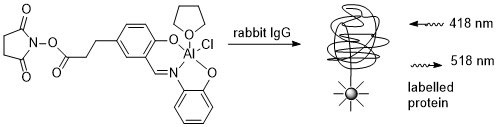
37. Towards Novel Biolabels: Synthesis of a Tagged Highly Fluoresent Schiff-Base Aluminium Complex
M. S. J. Briggs, J. S. Fossey, C. J. Richards, B. Scott and J Whately, Tetrahedron Lett. 2002, 43, 5169.
36. Simple Phosphinite-Oxazoline Ligands for Asymmetric Catalysis
G. Jones and C. J. Richards, Tetrahedron Lett. 2001, 42, 5553.
35. A Rapid Approach to Ferrocenophanes via Ring-Closing Metathesis
A. J. Locke, C. Jones and C. J. Richards, J. Organomet. Chem. 2001, 637 – 639, 669.
34. Synthesis of a C3-Symmetric Ferrocenylphosphine and its Application to the Suzuki Reaction of Aryl Chlorides
T. E. Pickett and C. J. Richards, Tetrahedron Lett. 2001, 42, 3767.
33. Metallocene Appended Imidazoles Displaying Virtual Planar Chirality
G. Jones and C. J. Richards, Organometallics 2001, 20, 1251.
[See also: https://sciforum.net/paper/view/1873]
32. Diastereoselective Synthesis of Enantiopure C2-Symmetric Dihaloferrocenes
A. J. Locke, T. E. Pickett and C. J. Richards, Synlett 2001, 141.
31. Planar Chiral Mimetics. A New Approach to Ligand Design for Asymmetric Catalysis
G. Jones, D. C. D. Butler and C. J. Richards, Tetrahedron Lett. 2000, 41, 9351.
[See also: https://sciforum.net/paper/view/1873]
30. Cationic 2,6-Bis(2-oxazolinyl)phenylpalladium(II) Complexes: Catalysts for the Asymmetric Michael Reaction
M. A. Stark, G. Jones and C. J. Richards, Organometallics 2000, 19, 1282-1291.
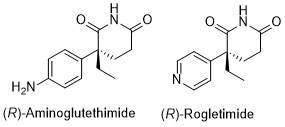
29. Production of (R)-Aminoglutethimide: A New Route from 1-Chloro-4-nitrobenzene
M. J. Bunegar, U. C. Dyer, G. R. Evans, R. P. Hewitt, S. W. Jones, N. Henderson, C. J. Richards, S. Sivaprasad, B. M. Skead, M. A. Stark and E. Teale, Org. Pro. R. & D. 1999, 3, 442.
28. The First Example of an Enantiopure Planar Chiral Hydroxyferrocene Ligand
T. E. Pickett and C. J. Richards, Tetrahedron: Asymmetry 1999, 10, 4095.
27. Assignment of 1H NMR Chemical Shifts in 1,2- and 1,1′-Disubstituted Ferrocenes
T. E. Pickett and C. J. Richards, Tetrahedron Lett. 1999, 40, 5251.
26. The Asymmetric Synthesis of [3](1,1′)- and [3](1,1′)[3](3,3′)-Ferrocenophane
A. J. Locke and C. J. Richards, Organometallics 1999, 18, 3750.
25. Synthesis and Highly Diastereoselective Palladation of (η5-(S)-2-(4-methylethyl)oxazolinylcyclopentadienyl)(η4-tetraphenylcyclobutadiene)cobalt
A. M. Stevens and C. J. Richards, Organometallics 1999, 18, 1346.
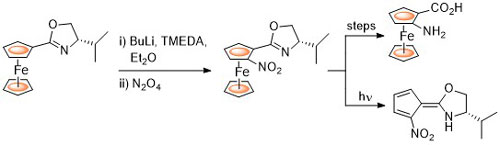
24. 2-Nitroferrocenyloxazolines: Precursors to Nitrofulvenes and Derivatives of (pS)- and (pR)-2-Aminoferrocenecarboxylic Acids
R. Salter, T. E. Pickett and C. J. Richards, Tetrahedron: Asymmetry 1998, 9, 4239.
23. Recent Advances in the Generation of Non-Racemic Ferrocene Derivatives and their Application to Asymmetric Synthesis. Review
C. J. Richards and A. J. Locke, Tetrahedron: Asymmetry 1998, 9, 2377.
22. Synthesis and X-Ray Crystal Structure Analysis of a Scalemic C2-Symmetric Ferrocenophane
A. J. Locke, C. J. Richards, D. E. Hibbs and M. B. Hursthouse, Tetrahedron: Asymmetry 1997, 8, 3383.
21. A Metallocene Molecular Gear.
A. M. Stevens and C. J. Richards, Tetrahedron Lett. 1997, 38, 7805.
20. Catalysts for Carbon-Carbon Bond Formation
M. A. Stark and C. J. Richards, Patent application 9709342.1, May 1997.
19. Synthesis and Application of Cationic 2,6-Bis(2-oxazolinyl)phenylpalladium(II) Complexes
M. A. Stark and C. J. Richards, Tetrahedron Lett. 1997, 38, 5881.
18. Organoiron Chemistry 1: Ferrocene and Dienyl Iron Tricarbonyl Cation Chemistry
C. J. Richards in Transition Metals in Organic Synthesis, A Practical Approach, Ed.: S. E. Gibson, Oxford University Press, 1997, pp 65-97.
17. Asymmetric Synthesis of 1- and 1,1′-Ferrocenepropanoic Acids
A. J. Locke and C. J. Richards, Tetrahedron Lett. 1996, 37, 7861.
16. Synthesis of Phosphinoferrocenyloxazolines. New Ligands for Asymmetric Catalysis
C. J. Richards and A. W. Mulvaney, Tetrahedron: Asymmetry 1996, 7, 1419.
15. Addition of Silyl Enol Ethers and Silyl Ketene Acetals to Ferrocenylmethyl Ethers. Synthesis of Precursors to Chiral Bridged Ferrocenophanes
A. J. Locke, N. Gouti, C. J. Richards, D. E. Hibbs and M. B. Hursthouse, Tetrahedron 1996, 52, 1461.
14. PdCl2-Complexes Containing Phosphinoferrocenyloxazoline Ligands. X-ray Crystal Structure Analysis and Application to Grignard Cross-Coupling
C. J. Richards, D. E. Hibbs and M. B. Hursthouse, Tetrahedron Lett. 1995, 36, 3745.
13. Synthesis of 2-[2-(Diphenylphosphino)ferrocenyl]oxazoline Ligands
C. J. Richards, T. Damalidis, D. E. Hibbs and M. B. Hursthouse, Synlett 1995, 74.
12. Stereoselective Addition of Silyl Enol Ethers to a-Ferrocenylcarbenium Ions
C. J. Richards, D. E. Hibbs and M. B. Hursthouse, Tetrahedron Lett. 1994, 35, 4215.
11. Enantioselective Diels-Alder Reactions of Enals: Fighting Species Multiplicity of the Catalyst with Donor Solvents
D. Sartor, J. Saffrich, G. Helmchen, C. J. Richards and H. Lambert, Tetrahedron: Asymmetry 1991, 2, 639.
10. The Transition Elements. C. J. Richards and S. E. Thomas in General and Synthetic Methods Volume 13, Ed.: G. Pattenden, 1992, pp 250-284.
9. Synthesis and Reactivity of Iron Tricarbonyl Complexes of Vinylketenes, Vinylketenimines and Vinylallenes
L. Hill, C. J. Richards, S. P. Saberi and S. E. Thomas, Pure Appl. Chem. 1992, 64, 371.
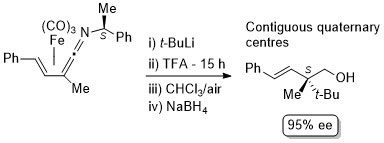
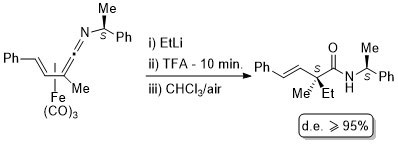
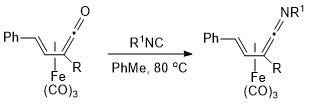
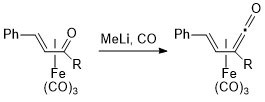
8. Nucleophilic Addition to Vinylketenimine Complexes. The Asymmetric Synthesis of Carbon Quaternary Centres
C. J. Richards and S. E. Thomas, Tetrahedron: Asymmetry 1992, 3, 143.
7. Structure of a Chiral Amide Derived from a (Vinylketenimine)tricarbonyliron(0) Complex
N. W. Alcock, C. J. Richards and S. E. Thomas, Acta Cryst. 1991, C47, 1261.
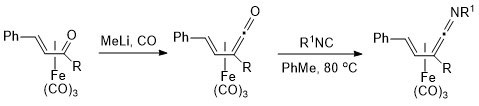
6. Preparation of Tricarbonyl(η4-vinylketene)iron(0) Complexes from Tricarbonyl(η4-vinyl ketone)iron(0) Complexes and Their Subsequent Conversion to Tricarbonyl(η4-vinylketenimine)iron(0) Complexes
N. W. Alcock, C. J. Richards and S. E. Thomas, Organometallics 1991, 10, 231.
5. Transition metal-stabilised vinylketenes
L. Hill, C. J. Richards and S. E. Thomas, Pure Appl. Chem. 1990, 62, 2057.
4. Generation of Homochiral Quaternary Carbon Centres from (Vinylketenimine)-tricarbonyliron(0) Complexes
N. W. Alcock, G. A. Pike, C. J. Richards and S. E. Thomas, Tetrahedron: Asymmetry 1990, 1, 531.
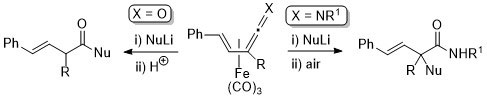
3. Nucleophilic Addition to Vinylketene- and Vinylketenimine (Allenylideneamine)-tricarbonyliron(0) Complexes
L. Hill, C. J. Richards and S. E. Thomas, J. Chem. Soc., Chem. Commun. 1990, 1085.
2. Conversion of (Vinylketene)tricarbonyliron(0) Complexes to (Vinylketenimine)tricarbonyliron(0) Complexes
C. J. Richards and S. E. Thomas, J. Chem. Soc., Chem. Commun. 1990, 307.
1. Conversion of α,b-Unsaturated Ketone Complexes into α,-Unsaturated Ketene Complexes
N. W. Alcock, T. N. Danks, C. J. Richards and S. E. Thomas, J. Chem. Soc., Chem. Commun. 1989, 21.