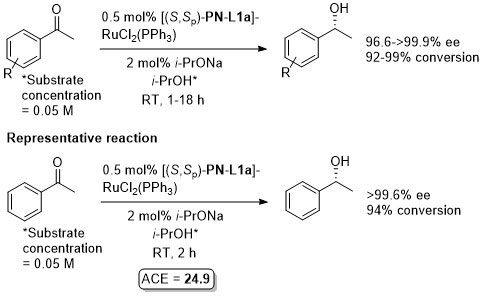
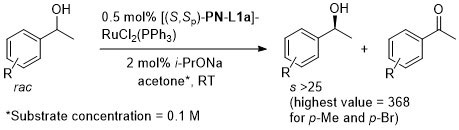
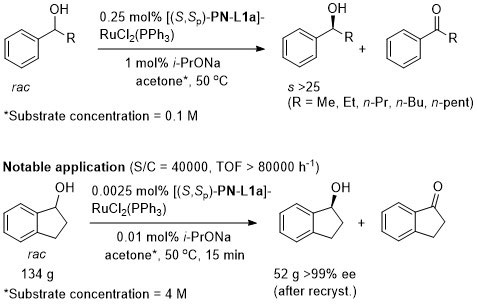
In situ generated catalyst utilised first by Sammakia [JOC97-62-6104] and Hou [CJC98-16-117]. Isolated catalyst prepared by Uemura [JOMC99-572-163]. The following lists reactions for which the application of these ligands has resulted in >80% ee. ACE = Asymmetric Catalytic Efficiency. Literature covered until end 2017.
Ru – Transfer hydrogenation Orgmet99-18-2291
Method also applied to the reduction of alkyl methyl ketones RCOMe with opposite S enantioselection (R = t-Bu >99% ee, R = Cy 66% ee, R = n-hexyl 26% ee). The example of the representative reaction, under the same conditions, has been reported to give 96% ee, 99% conversion, see: Orgmet12-31-4241.
Ru – Oxidative kinetic resolution Orgmet99-18-2291
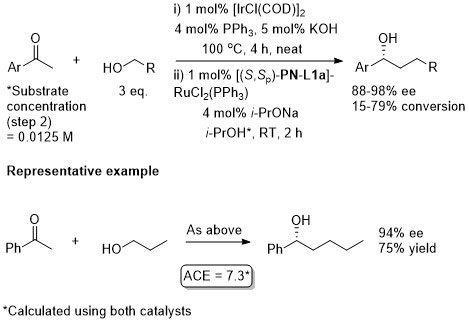
Ru – Reductive alkylation Angew06-45-3819
A one-pot sequential sequence also requiring [IrCl(COD)]2.
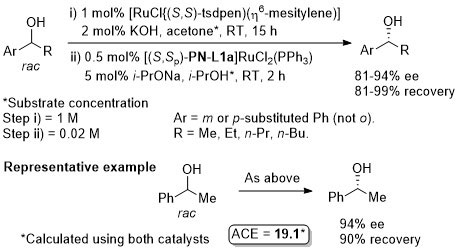
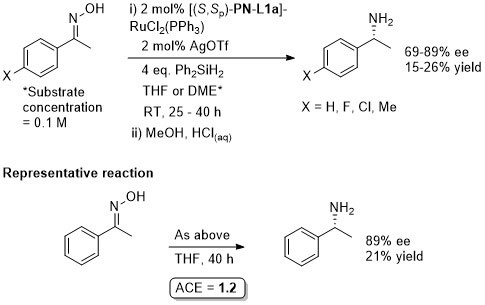
Ru – Deracemisation AJC07-2-393
A one-pot sequential sequence also requiring [RuCl{(S,S)-tsdpen)(η6-mesitylene)].
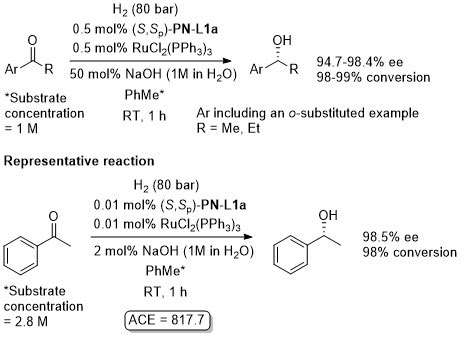
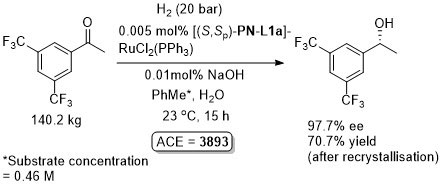
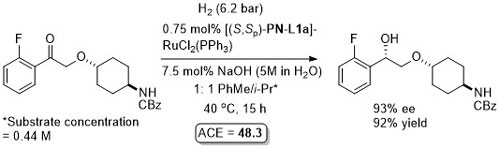
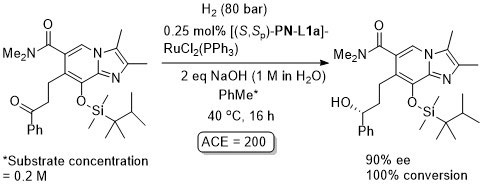
Ru – Hydrogenation
Both in situ generated and isolated [(S,Sp)-PN-L1a]RuCl2(PPh3) have been applied to hydrogenation with essentially identical results. An induction time has been reported on use of the isolated complex. In contrast to transfer hydrogenation, significant rate enhancements are possible by increasing the pressure of H2.
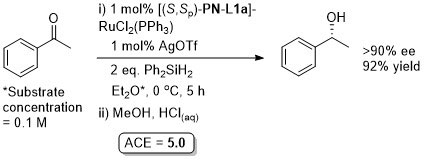
Ru – Hydrosilylation Orgmet98-17-3421